
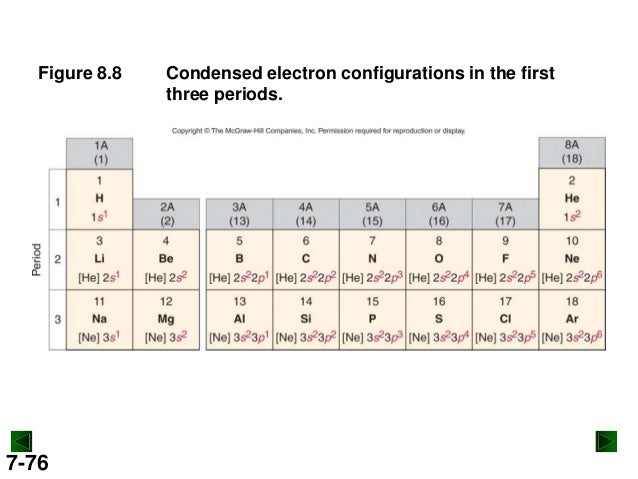
If there are more electrons than protons, the ion has a negative charge and is called an anion.Įlements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). can be written using the period table or an electron configuration chart. helps chemist understanding how elements form chemical bonds. If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. The calcium atom contains 20 electrons, and the electronic configuration of calcium is 2,8,8,2.Ca also occurs in group 2 elements and one of the Alkaline earth metals in the periodic table. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons-or not.Īn ion of an atom is one in which the number of protons and electrons is not the same. The Calcium electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Remember, a neutral atom contains the same number of protons and electrons. The upper right side shows the number of electrons in a neutral atom. The element atomic number and name are listed in the upper left. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. Chemical Properties of Calcium Electrochemical Equivalent: 0.7477g/amp-hr Electron Work Function: 2.87eV Electronegativity: 1 (Pauling) 1.04 (Allrod Rochow). The electron shells are shown, moving outward from the nucleus. The electron configuration is the standard notation used to describe the electronic structure of an atom.
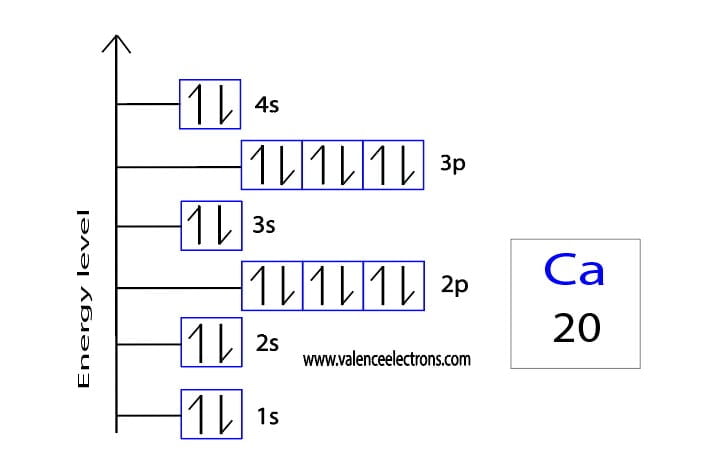
Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.įor each electron shell atom diagram, the element symbol is listed in the nucleus. To write the orbital diagram for the Calcium atom (Ca) first we need to write the electron configuration for just Ca. Electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 2. This list of electron configurations of elements contains all the elements in increasing order of atomic number. The sum of these superscripts should equal the atomic number for a neutral atom.
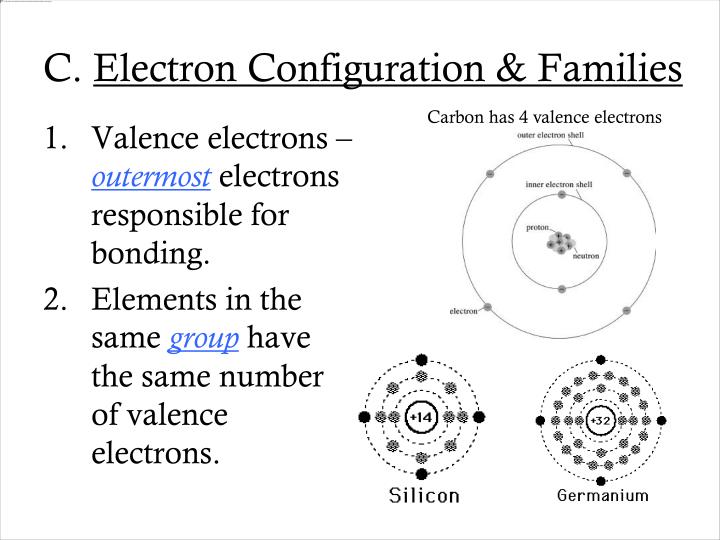
The superscripts represent the electrons present in each region of the periodic table. Note: The unabbreviated electron configuration of argon is Ne 3s 2 3p 6. The electron configuration for Gallium, Ga is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 Gallium, Ga has 31 protons and 31 electrons. Therefore, the argon complete electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6. For that, we have electron shell diagrams. Ca: calcium: 180: 194: 231: 171 133: 197 21: Sc: scandium: 160: 184: 211. The electron configuration shows the distribution of electrons into subshells. Then two electrons will enter the 3s orbital of the third orbit and the remaining six electrons will be in the 3p orbital. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.


 0 kommentar(er)
0 kommentar(er)
